| ? | ? | ? |
Anatomy of a battery
An electrochemical cell consists of two half-cells, each consisting of an electrode and an electrolyte. Differences in redox potential between the two generates electrical current as they react. The two electrolytes may be the same or different substances, but must be electrically connected so that charged particles can flow from one to another.
Fascinating! Thank you Wikipedia.
Here's the good bit: a battery consists of one or more electrochemical cells. This information was sufficient excuse to find a hammer and chisel and raid the recycling.
Lantern Battery
The lantern battery was rated at 6 V, but the zinc-carbon chemistry generated a potential of 1.5 V. Very likely it contained four cells, or a multiple of four. A meaty blow provided evidence for the theory.
The springy contacts were saved to make a battery charger. I could have kept the zinc case to make killed spirits (zinc chloride flux), but I forgot. The carbon electrodes were extracted for further amusement, but what?
4 things to do with a stick of graphite
- Make pointy with pencil sharpener, add high current AC, strike an arc: braze and gouge sheet metal, or electrocute yourself in a blast of EMI.
- Invest in paper, beret; sketch nude model.
- Dip in a zinc can full of electrolyte and power your radio. Oh, wait.
- Electrolyse dilute sulphuric acid, make oxygen bubbles for that homebrew etch tank. Aha!
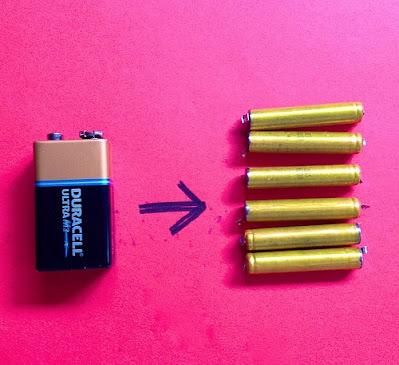
Rechargeable PP3
Fully juiced, this was once capable of 8.4 V. A single NiMH cell generates 1.2 V so there was probably 7 cells inside. Readers of a mathematical bent will note that this number is greater than 6! Therefore this rechargeable battery contains even more cells than the alkaline equivalent. Amazing.
An unfortunate number of them were covered in sticky brown gunk which I had no interest in investigating. I suspected it was leaked electrolyte of dire toxicity. Some or all of the cells might still have functioned. An energetic roboticist needing a compact, low capacity rechargeable could whip up a trickle charger out of a 317T. I looked around the room and couldn't see such a person, so it went in a plastic bag in the not-for-children corner of the shed.
The battery clip was clearly beyond recycling.
Laptop Battery
Last on the list was this 14.4 V 5200 mAh Li ion rechargeable laptop battery. It died a long slow death some time ago. I took a screwdriver to it in an utterly reckless manner. It bust open with a puff of smoke as I obliterated a small PCB inside. Whoops! That was probably the overvoltage protection circuit.
Inside I was chuffed to find, count them, eight 18650 Li ion cells. As I pulled them out, paying no particular attention to what I was doing, there were a couple of sparks. Maybe some of these were worth having! Seven of the cells registered around 0.4 V 4.0 V, the other exactly 0 V. There is a possibility that only that one cell is utterly destroyed, and the others may have some life in them yet (hope springs eternal). I will try out my homebrew TP4056 charger on them, and if any of the cells hold a charge they might find a home in my DIY bike lights powering a 10 W Cree LED.
Hack A Day
Linkback
Hack A Day











5 comments:
Some rechargeable PP3 batteries have seven rechargeable AAAA cells, rather than those blocks. It kind of sounds weird because you'd expect only 6 of these cells to fit inside on PP3, but in fact there's a seventh sandwiched in there.
In some 6v lantern batteries, I have found D cells
packaged as if you had bought them from a store. If I am remembering right, these had a spacer just like the spacer that came with my 6v lantern!
Hacksaw down the middle of a 9 volt . Much easier
Prise the plastic top from a 12 V 7 amp hour and you will more than likely find the acid has evaporated so you chuck it away and buy a new one. If you tricle sulphuric acid down a toothpick you can refil them
You can always use a Lithium Battery Charger =)
like this one
http://dx.com/p/trustfire-tr002-lithium-battery-charger-for-10440-14500-17670-18500-18650-100-240v-22577
I use it to charge some 18500 batteries from an old laptop battery and works fine on my TR1200 flashlight
Save a couple carbon rods out of the lantern battery and store in emergency kit for the car. Connected via jumper cable to the car battery, it creates a pseudo spot welder / soldering iron. I used it once to repair a broken terminal on a starter motor, getting the car back on the road
Post a Comment